Clear Guide Medical proudly announces the FDA clearance for the CLEAR GUIDE SCENERGY computer aided instrument guidance system, alongside the new TP Access Tool with SteriGRID™.
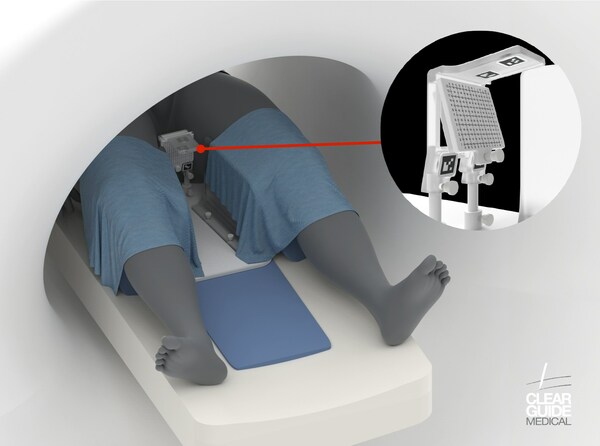
CLEAR GUIDE SCENERGY is a state-of-the-art system that delivers remarkable accuracy to enhance image-guided diagnostic and interventional procedures. Leveraging image fusion, instrument recognition and tracking, multi-modal markers and target planning functionalities, the system provides clinicians with a solution for in-suite MR guided transperineal procedures.
“We are thrilled about the evolution of the SCENERGY product. While we always believed it would have a profound impact on cancer screenings, the clinical results for transperineal interventions have been truly remarkable,” says Clear Guide Medical CMO Dr. Nick Karahalios.
“This latest FDA clearance exemplifies our commitment to advancing medical technologies for the benefit of patients and healthcare professionals,” says Clear Guide Medical COO Patrick Duke.
The FDA’s determination underscores the device’s compliance with regulatory standards, paving the way for its application in transperineal interventions as a new standard of care. This new application is a testament to CGM’s dedication to tailoring their patented technologies to meet emerging clinical needs.
For transperineal biopsy procedures, the CLEAR GUIDE SCENERGY-TP allows clinicians to guide biopsy needles through the perineal skin into the prostate, minimizing the risk of contamination associated with transrectal biopsies. According to the Mayo Clinic, this approach substantially eliminates the risk of sepsis to compared to the risk associated with transrectal biopsy methods.