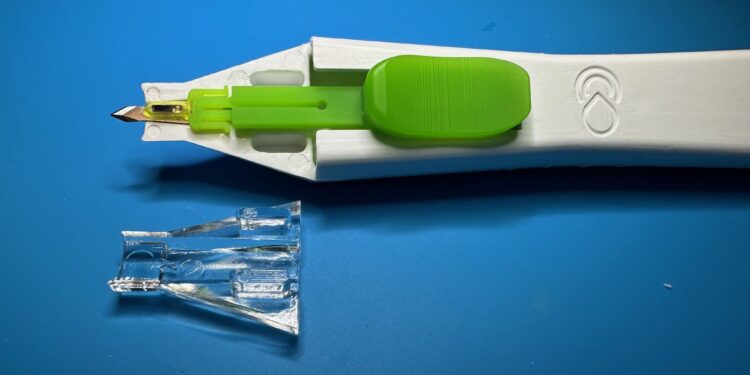
Injectsense, a sensor-enabled digital health company, today announced the successful completion of the first human implant and wireless intraocular pressure (IOP) measurements using its FDA breakthrough-designated, ultraminiature implantable sensor.
The IOP-Connect system study is led by Dr. Juan Mura of the Centro de la Vision in Santiago, Chile. Dr. Mura performed the implant procedure, demonstrating the feasibility of the minimally invasive, suture-less delivery. The team collected IOP data using an external reader. Following the successful first implant, the trial is continuing with a target enrollment of 20 subjects.
“From a physician’s perspective, this is a major step toward understanding IOP fluctuations and providing more effective glaucoma treatment,” said Dr. Juan Mura, MD, Centro de La Vision. “It’s extremely exciting to have a direct path to an implantable sensor that will provide autonomous measurement 24/7.”
“Although safety is the primary focus of this study, we are also informing our product design to meet the expectations of ophthalmologists across the globe,” said Ariel Cao, President/CEO of Injectsense Inc. “We expect these sensors to be easily delivered in the doctor’s office.”
The next steps for the company include fine tuning of the delivery tool and incorporating the Injectpower SAS solid-state microbattery into the sensor for autonomous and continuous measurement of IOP from inside the eye. The final product platform will use “smart glasses” worn by patients for a few minutes each week that will wirelessly recharge the sensor and securely upload sensor-stored data to a smart phone and the cloud.